Gamida Cell Reports Preliminary Data from Phase 1 Study of Natural Killer (NK) Cell Therapy Candidate GDA-201
Data show promising early evidence of anti-tumor activity in patients with relapsed/refractory B cell non-Hodgkin lymphoma
Full data readout of Phase 1 study expected in Q1 2024
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231016887701/en/
(Graphic:
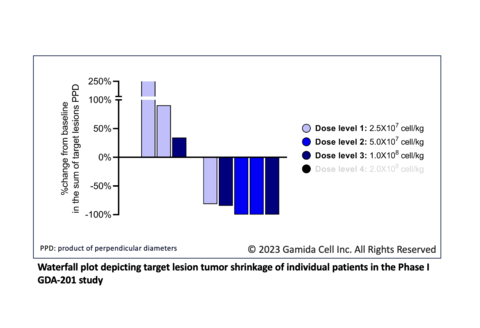
Enrolled patients were heavily pretreated with a median of six prior lines of therapy, including CAR-T cell therapy (six patients) and hematopoietic stem cell transplant (four patients). Preliminary results showed marked shrinkage of target lesions in five patients; efficacy evaluation showed two patients with complete response, two with partial response, and one with stable disease. No dose-limiting toxicities were reported in the 10 patients treated with doses up to 1x108 cells/kg GDA-201 in combination with rituximab.
Activity appears to be dose dependent with two of the three patients in Cohort 3 responding. The fourth and final cohort of the study, at the target dose level of 2x108 cells/kg, is currently enrolling.
“We have demonstrated that our nicotinamide (NAM)-modified NK cells have enhanced metabolic fitness, resistance to oxidative stress and potent cytotoxicity, meaning that GDA-201 has the potential for powerful anti-tumor activity," said
The 10 enrolled patients were diagnosed with diffuse large / high grade B cell lymphoma (6), marginal zone lymphoma (2), follicular lymphoma (1) and mantle cell lymphoma (1). Successive cohorts of patients received dose levels of 2.5x107 cells/kg, 5x107 cells/kg and 1x108 cells/kg of GDA-201 with rituximab after fludarabine/cyclophosphamide lymphodepletion. Two patients treated had cytokine release syndrome (grade 1 and grade 2, respectively). The most common grade 3-4 adverse event was transient neutropenia. There were no reported cases of immune effector cell associated neurotoxicity syndrome or graft versus host disease. There was one death from progressive disease.
The NK cells which comprise GDA-201 are powered by Gamida Cell’s proprietary NAM technology, which enhances and expands cells to enhance functionality and phenotype, increase metabolic fitness and reduce oxidative stress. These functional qualities were studied in detail in a recent study published in
Additionally, the publication includes clinical data from 19 non-Hodgkin lymphoma patients who received the fresh formulation of GDA-201 in a Phase 1 study conducted at the
About GDA-201
GDA-201 is an intrinsic NK cell therapy candidate being investigated for the treatment of hematologic malignancies. A multicenter Phase 1 study of GDA-201 for the treatment of non-Hodgkin lymphoma is ongoing (NCT05296525). Results are expected in Q1 2024.
GDA-201 is an investigational cell therapy candidate, and its safety and efficacy have not been established by the FDA or any other health authority.
About
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including with respect to the Company’s cell therapy candidate, GDA-201. Any statement describing Gamida Cell’s goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to a number of risks, uncertainties and assumptions including those related to clinical, scientific, regulatory and technical developments and those inherent in the process of developing and commercializing product candidates that are safe and effective for use as human therapeutics. In light of these risks and uncertainties, and other risks and uncertainties that are described in the Risk Factors section and other sections of Gamida Cell’s Quarterly Report on Form 10-Q filed with the
OMISIRGE™ is a trademark of
View source version on businesswire.com: https://www.businesswire.com/news/home/20231016887701/en/
Media Contact:
Orangefiery
media@orangefiery.com
1-818-209-1692
Investor Contact:
Chuck@lifesciadvisors.com
1-646-627-8390
Source:
